Abstract
Background: Fixed-duration treatment of chronic lymphocytic leukemia (CLL) with venetoclax combined with a BTK-inhibitor(i) results in prolonged progression free survival (PFS). Undetectable levels of minimal residual disease (uMRD) reach an early plateau and are highly predictive for PFS in case of venetoclax in combination with anti-CD20, but kinetics are different when combined with a BTK-i. MRD-levels mirror disease activity in blood (PB) and bone marrow but much less of lymph node (LN) disease, the suspected critical site of CLL proliferation and development of resistance. Although regular CT-scans do provide information on tumor-bulk, there is a high uncertainty concerning the bio-clinical significance of the frequently observed minimally enlarged LN.
The NEXT-STEP trial evaluates the efficacy of adding 6 cycles ibrutinib and obinutuzumab for treatment naive patients who do not achieve complete remission (CR) and uMRD after 3 cycles of ibrutinib lead-in and 12 cycles of combined ibrutinib and venetoclax. The first preplanned interim analysis evaluates safety and efficacy for the first 30 eligible patients until the end of cycle 8. In addition, exploratory analyses of 18F-fluorodeoxyglucose (FDG) PET-CT as well as presence of CLL cells in residual LN by fine-needle aspirations (FNA) were performed. We here present this interim analysis.
Methods: HOVON 158/NEXT STEP trial is an open-label, single arm phase 2 study. Eligible patients with untreated CLL or small lymphocytic lymphoma (SLL) receive three 28 days cycles of ibrutinib followed by 12 cycles of combined ibrutinib and venetoclax. IwCLL response and MRD bone marrow are assessed after cycle 15. Patients achieving CR with uMRD4 (8-colour flow cytometry, <10-4) stop treatment. Intensification with an additional 6 cycles of ibrutinib combined with obinutuzumab is provided for patients with MRD positivity and/or less than CR. After cycle 8 an exploratory analysis is done with locally assessed Deauville score (DS) on PET-CT. In addition, if at end of cycle 8 a LN > 1.5 cm is present on physical examination or CT scan and located cervical, axillar or inguinal (for safe accessibility), a fine needle aspiration (FNA) by ultrasound is requested. Obtained cells are centrally analyzed by flow cytometry.
Results: Median age was 69 years (range 34-85), 70% were male. One patient had SLL. CIRS score > 6 was found in 6 patients (20%). Median eGFR was 65 ml/min (range 39-132). Median lymphocyte count was 157.3 x 109/l (range 2.50-475). CLL-IPI score was low and intermediate in one (3%) and 6 (20%) and high and very high in 18 (60%) and one (3%) patients, respectively. Del (17p)/TP53 mutation was found in 3 patients (10%). Genomic complexity (≥ 3 aberrations) was found in 3 patients (10%).
Overall response rate based on PB and CT scan after cycle 8 was 100%. Levels of responses are depicted in table 1. One patient discontinued therapy during the first 8 cycles, due to adverse events. No progression events or death have occurred and no new safety signals were reported at interim analysis.
Of all the PET-scans at baseline 13 (43%) demonstrated an increased uptake (DS4) and 5 (17%) uptake > mediastinal blood pool (DS3). In all but one patient DS decreased after 8 cycles; 1 (3%) remainded DS4, 2 (7%) had DS3, 9 (31%) had uptake equal or below mediastinal blood pool (DS2) and 16 (55%) had no uptake (DS1). In 1 patient PET was not performed.
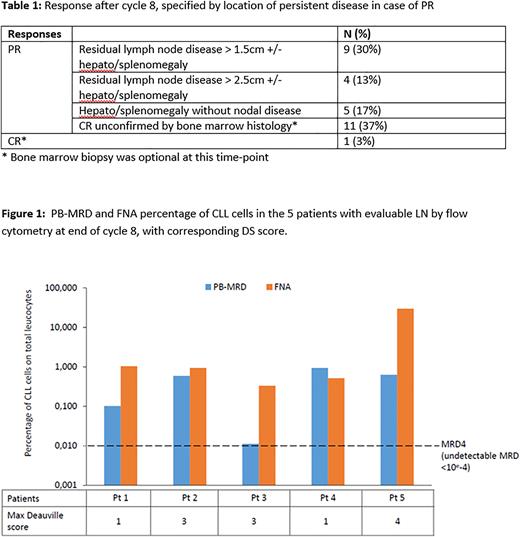
Of 13 patients with enlarged LN after cycle 8, FNA could be done in 9 patients, of which 5 contained sufficient number of cells for analysis. FNA was performed in all 3 patients with DS3-4 because of remaining enlarged LN. In all cases, persistent nodular CLL cells were detected by flow cytometry. In 4 of the 5 patients, MRD levels were higher in the LN as compared to PB. The patient with DS4 demonstrated the highest CLL cells percentage in FNA (Figure 1).
Conclusion: Three cycles of ibrutinib followed by 5 cycles of ibrutinib and venetoclax results in tumor bulk reduction with a decrease in metabolic uptake on PET-CT in all but one patient. We demonstrated persistent CLL cells by flow cytometry in enlarged LN, suggesting residual LN serve as a sanctuary of CLL.
Disclosures
Levin:AbbVie, Roche and Janssen: Other: Travel expenses. Niemann:Genmab: Consultancy; Takeda: Consultancy; Octapharma: Consultancy; CSL Behring: Consultancy; Roche: Consultancy; Novo Nordisk Foundation: Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Kater:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Patents & Royalties: pending, Research Funding; Roche/genentech: Membership on an entity's Board of Directors or advisory committees; LAVA: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: pending; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal